Know the Risks and Benefits of Medical Devices
Medical science continues to make great progress in diagnosing illnesses and treating them. Devices of many types allow doctors to see inside the body to diagnose a variety of conditions. Other types of medical devices that are used or implanted inside the body provide relief from many painful conditions. However, no matter how far medical science has progressed, there are still risks and benefits that people need to consider before undergoing even the most routine procedure involving a medical device.
Here are a few common devices and procedures, as well as some injuries and issues that have been associated with them.
Medical Scopes
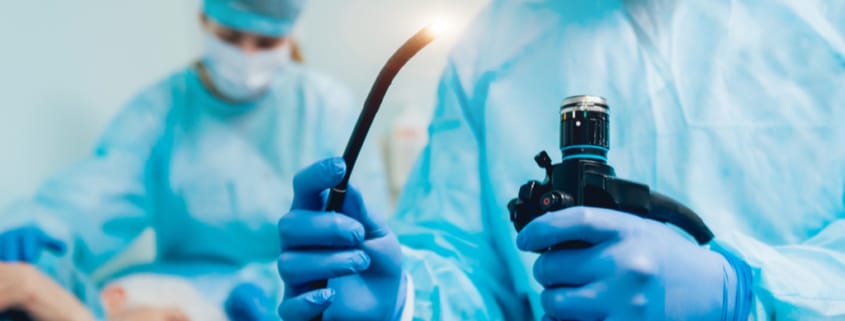
Each year, millions of people in the U.S. have a procedure with a medical scope of some type. In these procedures, a doctor places an instrument called an endoscope inside the body to examine the esophagus, stomach, bile ducts, colon, lungs, bladder, or other organs. After each procedure these devices are cleaned and re-used on another patient. Duodenoscopes, which are used to diagnose and treat stomach and other GI tract conditions, have been of particular concern.
Since 2013, at least 35 people have died from after having a procedure that used a duodenoscope. The problem is that the design of the scope makes cleaning it extremely difficult, so even if the scope is cleaned according to the manufacturer’s instructions, bacteria can still be trapped inside the scope and transferred to another patient. A paper published in April 2018 showed that 71 percent of scopes tested at three major U.S. hospital were contaminated with bacteria.
In 2015 the FDA directed three duodenoscope manufacturers (Olympus, Fujifilm, and Pentax) to study this issue. Interim results of these studies released in December 2018 showed that up to three percent of samples were contaminated with “low-concern” organisms, and up to three percent were contaminated with organisms of high concern. Sampling is continuing.
One manufacturer, Olympus, pled guilty in December 2018 to charges of failing to file reports about infections stemming from its scopes. The company agreed to pay $85 million to resolve the charges. Additionally, the company settled lawsuits with two women whose husbands died after undergoing a procedure with the scope and becoming infected with a “superbug” bacteria resistant to most antibiotics.
Other types of scopes are similarly difficult to clean. Bronchoscopes, which are used to look inside the lungs, have also been found to be contaminated, even after cleaning, and have likewise been linked to “superbug” infections.
Bone Cement
There are over a million joint replacements performed each year in the U.S. In many of these procedures, the surgeon uses bone cement. Bone cement doesn’t actually “glue” the joint into place; it fills the space between the artificial joint and the bone to keep the joint in place. The use of bone cement in joint replacements is not without complications, however. The bone cement can break loose, which can cause pain and affect movement. It can also leak into the body.
The most serious problem is bone cement implantation syndrome (BCIS). Fortunately, this complication is rare, but when it happens, it causes the cardiovascular system to collapse and can cause death, often during the procedure. BCIS has been reported with knee, shoulder, and hip replacements. One study from the UK reported 41 deaths linked to the use of cement in hip replacement.
BCIS has also been reported in patients undergoing spinal surgery. As early as 2002, the FDA sent a warning about a bone cement that was being used to treat spinal fractures and had been linked to serious complications, including blood clots in the lungs, cardiac failure, and death.
In 2011 the government filed charges against a company called Synthes, a manufacturer of bone cement. The company had been testing a cement that had not been approved by the FDA for use in spinal surgeries. Five patients who received the cement had died during surgery after the company ignored reports that the cement could have fatal consequences. Because the company had not been conducting a clinical trial, patients weren’t advised of the risks and given a chance to decide if they wanted to receive the bone cement. Four Synthes executives were also charged, pled guilty and sentenced to time in jail.
Pelvic Mesh
Pelvic mesh is a relatively recent product that was initially marketed in the 1990s to treat stress urinary incontinence in women. After the success of that product, it started being used for pelvic organ prolapse. However, the use of the mesh for pelvic organ prolapse has been associated with complications including pain, infection, bleeding, mesh erosion, urinary problems, and perforation of organs, and has prompted tens of thousands of lawsuits.
The FDA received over 10,000 reports of serious injuries from pelvic (transvaginal) mesh that occurred from January 2008 to October 2018, including 77 deaths. Previously, in 2011, the FDA had issued a warning, and in 2016 they classified it as a high-risk device.
Earlier this year, a woman was awarded $41 million in a verdict against one of the manufacturers of pelvic mesh, Johnson & Johnson. Altogether, more than 100,000 women are suing manufacturers of pelvic mesh due to the complications they have experienced. Some women describe continuous pain due to the plastic of the mesh perforating the vaginal wall.
This is another instance of the product being marketed and used without the benefit of long-term study, to the detriment of thousands of women. Transvaginal mesh and is banned in the UK, Australia and New Zealand. On February 12, 2019, an FDA panel met to discuss the future of transvaginal mesh in the U.S. The panel decided that rather than pulling the mesh off the market, they would like more long-term studies.
What to Do If You Need a Procedure or Medical Device
If you are faced with needing a medical procedure or device, how can you weigh the risks and benefits? You need to be fully aware of whether the device has been studied in clinical trials and has been approved by the FDA. Even if a device has been approved , such as a duodenoscope, you need to know if there are other issues, such as infection risk, associated with its use.
Doing an internet search on the device or procedure may help answer questions such as:
- What is the history of the procedure/device?
- Has the device been approved by the FDA?
- What are the risks and benefits?
- What complications have been reported?
- Are there ongoing lawsuits associated with the device?
- What are the alternatives?
You should also carefully read the consent document for the procedure, as it should list the potential risks and complications.
Speak with your healthcare providers also. What is their experience with the procedure/device? How many times have they done the procedure? What are the most common complications? Before making a decision, you may also want to speak with other patients who have had the procedure.
The bottom line is that you should arm yourself with as much knowledge as you can before undergoing a procedure so you know the risks and benefits and can make an informed decision.